What Is The Makeup Of Carbs
What are Carbohydrates?
Carbohydrates are a grouping of naturally occurring carbonyl compounds (aldehydes or ketones) that as well contain several hydroxyl groups. It may also include their derivatives which produce such compounds on hydrolysis. They are the most abundant organic molecules in nature and are also referred to as "saccharides". The carbohydrates which are soluble in water and sweetness in taste are chosen "sugars".
Construction of Carbohydrates
- Carbohydrates consist of carbon, hydrogen, and oxygen.
- The full general empirical structure for carbohydrates is (CH2O)due north.
- They are organic compounds organized in the class of aldehydes or ketones with multiple hydroxyl groups coming off the carbon chain.
- The building blocks of all carbohydrates are elementary sugars chosen monosaccharides.
- A monosaccharide tin can exist a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose).
The carbohydrates can be structurally represented in any of the iii forms:
- Open chain structure.
- Hemi-acetal structure.
- Haworth structure.
Open chain structure – It is the long straight-chain form of carbohydrates.
Hemi-acetal structure– Here the 1st carbon of the glucose condenses with the -OH grouping of the fifth carbon to grade a ring structure.
Haworth structure– Information technology is the presence of the pyranose ring construction.
Properties of Carbohydrates
Physical Properties of Carbohydrates
- Stereoisomerism – Compound shaving the same structural formula only they differ in spatial configuration. Instance: Glucose has two isomers with respect to the penultimate carbon atom. They are D-glucose and Fifty-glucose.
- Optical Activity – It is the rotation of plane-polarized light forming (+) glucose and (-) glucose.
- Diastereo isomers – It the configurational changes with regard to C2, C3, or C4 in glucose. Example: Mannose, galactose.
- Annomerism – It is the spatial configuration with respect to the first carbon atom in aldoses and the second carbon cantlet in ketoses.
Chemic Properties of Carbohydrates
- Osazone formation: Osazone are saccharide derivatives when sugars are reacted with an excess of phenylhydrazine. eg. Glucosazone
- Benedict's test: Reducing sugars when heated in the presence of an brine gets converted to powerful reducing species known as enediols. When Benedict'south reagent solution and reducing sugars are heated together, the solution changes its color to orange-red/ brick ruby-red.
- Oxidation: Monosaccharides are reducing sugars if their carbonyl groups oxidize to requite carboxylic acids. In Bridegroom's test, D-glucose is oxidized to D-gluconic acid thus, glucose is considered a reducing carbohydrate.
- Reduction to alcohols: The C=O groups in open-chain forms of carbohydrates tin be reduced to alcohols past sodium borohydride, NaBHiv, or catalytic hydrogenation (H2, Ni, EtOH/H2O). The products are known as "alditols".
Properties of Monosaccharides
- Most monosaccharides take a sweetness gustatory modality (fructose is sweetest; 73% sweeter than sucrose).
- They are solids at room temperature.
- They are extremely soluble in water: – Despite their high molecular weights, the presence of large numbers of OH groups makes the monosaccharides much more water-soluble than nearly molecules of similar MW.
- Glucose can dissolve in minute amounts of water to brand a syrup (ane g / 1 ml H2O).
Classification of Carbohydrates (Types of Carbohydrates)

The unproblematic carbohydrates include single sugars (monosaccharides) and polymers, oligosaccharides, and polysaccharides.
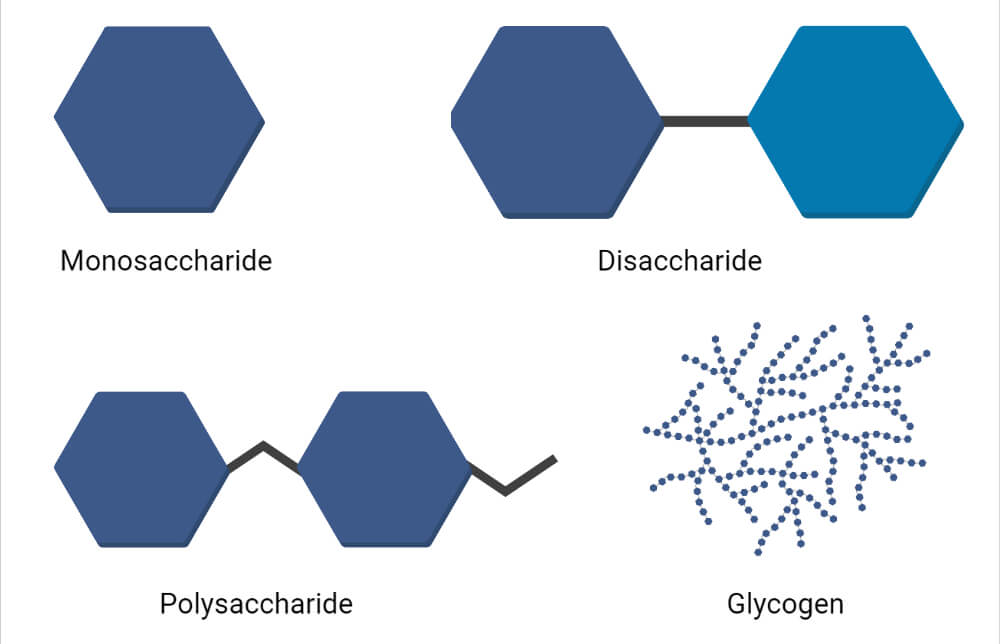
Monosaccharides
- The simplest group of carbohydrates and often called elementary sugars since they cannot be further hydrolyzed.
- Colorless, crystalline solids that are soluble in water and insoluble in a non-polar solvent.
- These are chemical compound that possesses a free aldehyde or ketone group.
- The general formula is Cnorth(H2O)northwardor Cdue northH2nOnorthward.
- They are classified co-ordinate to the number of carbon atoms they contain and also on the basis of the functional group present.
- The monosaccharides thus with 3, iv, 5, 6, 7 … carbons are chosen trioses, tetroses, pentoses, hexoses, heptoses, etc., and also every bit aldoses or ketoses depending upon whether they contain aldehyde or ketone grouping.
- Examples: Glucose, Fructose, Erythrulose, Ribulose.
Oligosaccharides
- Oligosaccharides are compound sugars that yield 2 to ten molecules of the same or different monosaccharides on hydrolysis.
- The monosaccharide units are joined by glycosidic linkage.
- Based on the number of monosaccharide units, information technology is further classified as a disaccharide, trisaccharide, tetrasaccharide, etc.
- Oligosaccharides yielding ii molecules of monosaccharides on hydrolysis is known as a disaccharide, and the ones yielding 3 or 4 monosaccharides are known as trisaccharides and tetrasaccharides respectively, then on.
- The full general formula of disaccharides is Cn(H2o)north-1and that of trisaccharides is Cn(H2O)n-two and then on.
- Examples: Disaccharides include sucrose, lactose, maltose, etc.
- Trisaccharides are Raffinose, Rabinose.
Polysaccharides
- They are also called "glycans".
- Polysaccharides contain more than than 10 monosaccharide units and can be hundreds of carbohydrate units in length.
- They yield more than 10 molecules of monosaccharides on hydrolysis.
- Polysaccharides differ from each other in the identity of their recurring monosaccharide units, in the length of their chains, in the types of bond linking units and in the degree of branching.
- They are primarily concerned with two important functions ie. Structural functions and the storage of free energy.
- They are further classified depending on the type of molecules produced as a result of hydrolysis.
- They may be homopolysaccharidese, containing monosaccharides of the aforementioned type or heteropolysaccharides i.e., monosaccharides of different types.
- Examples of Homopolysaccharides are starch, glycogen, cellulose, pectin.
- Heteropolysaccharides are Hyaluronic acid, Chondroitin.
Functions of Carbohydrates
Carbohydrates are widely distributed molecules in plant and beast tissues. In plants and arthropods, carbohydrates from the skeletal structures, they also serve every bit food reserves in plants and animals.They are important energy sources required for various metabolic activities, the energy is derived past oxidation.
Some of their major functions include
- Living organisms employ carbohydrates as accessible energy to fuel cellular reactions. They are the virtually abundant dietary source of free energy (4kcal/gram) for all living beings.
- Carbohydrates forth with beingness the principal energy source, in many animals, are instant sources of free energy. Glucose is broken downwardly by glycolysis/ Kreb's bike to yield ATP.
- Serve as energy stores, fuels, and metabolic intermediates. It is stored as glycogen in animals and starch in plants.
- Stored carbohydrates act as an free energy source instead of proteins.
- They class structural and protective components, similar in the cell wall of plants and microorganisms. Structural elements in the cell walls of bacteria (peptidoglycan or murein), plants (cellulose), and animals (chitin).
- Carbohydrates are intermediates in the biosynthesis of fats and proteins.
- Carbohydrates aid in the regulation of nerve tissue and is the energy source for the encephalon.
- Carbohydrates get associated with lipids and proteins to form surface antigens, receptor molecules, vitamins, and antibiotics.
- Formation of the structural framework of RNA and Deoxyribonucleic acid (ribonucleic acid and deoxyribonucleic acrid).
- They are linked to many proteins and lipids. Such linked carbohydrates are important in cell-cell communication and in interactions between cells and other elements in the cellular surround.
- In animals, they are an important constituent of connective tissues.
- Carbohydrates that are rich in fiber content assistance to prevent constipation.
- Too, they help in the modulation of the immune system.
References
- Lehninger, A. L., Nelson, D. 50., & Cox, G. G. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- Madigan, Thou. T., Martinko, J. Chiliad., Bender, K. Southward., Buckley, D. H., & Stahl, D. A. (2015). Brock biology of microorganisms (Fourteenth edition.). Boston: Pearson.
- Rodwell, 5. W., Botham, Thou. M., Kennelly, P. J., Weil, P. A., & Bough, D. A. (2015). Harper's illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
- https://biological science.tutorvista.com/biomolecules/carbohydrates.html
Source: https://microbenotes.com/carbohydrates-structure-properties-classification-and-functions/
Posted by: smithgert1936.blogspot.com

0 Response to "What Is The Makeup Of Carbs"
Post a Comment