Draw The Structure Of Beryllium Chloride Molecule
 | |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name Beryllium chloride | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.029.197 | ||
| PubChem CID |
| ||
| RTECS number |
| ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical formula | BeCl2 | ||
| Molar mass | 79.9182 g/mol | ||
| Appearance | White or yellow crystals | ||
| Density | 1.899 g/cm3, solid | ||
| Melting point | 399 °C (750 °F; 672 K) | ||
| Boiling point | 482 °C (900 °F; 755 K) | ||
| Solubility in water | 15.1 g/100 mL (20 °C) | ||
| Solubility | soluble in alcohol, ether, benzene, and pyridine slightly soluble in chloroform and sulfur dioxide | ||
| Structure | |||
| Crystal structure | hexagonal | ||
| Molecular shape | polymer | ||
| Thermochemistry | |||
| Heat capacity (C) | 7.808 J/K or 71.1 J/mol K | ||
| Std molar | 63 J/mol K | ||
| Std enthalpy of | −6.136 kJ/g or -494 kJ/mol | ||
| Gibbs free energy (Δf G˚) | -468 kJ/mol | ||
| Std enthalpy of | 16 kJ/mol | ||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 86 mg/kg (rat, oral) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[1] | ||
| REL (Recommended) | Ca C 0.0005 mg/m3 (as Be)[1] | ||
| IDLH (Immediate danger) | Ca [4 mg/m3 (as Be)][1] | ||
| Related compounds | |||
| Other anions | Beryllium fluoride Beryllium bromide Beryllium iodide | ||
| Other cations | Magnesium chloride Calcium chloride Strontium chloride Barium chloride Radium chloride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Chemical compound
Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its properties are similar to those of aluminium chloride, due to beryllium's diagonal relationship with aluminium.
Structure and synthesis [edit]
Beryllium chloride is prepared by reaction of the metal with chlorine at high temperatures:[2]
- Be + Cl2 → BeCl2
BeCl2 can also be prepared by carbothermal reduction of beryllium oxide in the presence of chlorine.[3] BeCl2 can be prepared by treating beryllium with hydrogen chloride.
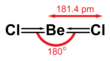
The solid is a 1-dimensional polymer consisting of edge-shared tetrahedra.[4] In contrast, BeF2 is a 3-dimensional polymer, with a structure akin to that of quartz. In the gas phase, BeCl2 exists both as a linear monomer and a bridged dimer with two bridging chlorine atoms where the beryllium atom is 3-coordinate.[5] The linear shape of the monomeric form is as predicted by VSEPR theory. The linear shape contrasts with the monomeric forms of some of the dihalides of the heavier members of group 2, e.g. CaF2, SrF2, BaF2, SrCl2, BaCl2, BaBr2, and BaI2, which are all non-linear.[5] Beryllium chloride dissolves to give tetrahedral [Be(OH2)4]2+ ion in aqueous solutions as confirmed by vibrational spectroscopy.[6]
Reactions [edit]
Beryllium chloride forms a tetrahydrate, BeCl2•4H2O ([Be(H2O)4]Cl2). BeCl2 is also soluble in some ethers.[7] [8]
Applications [edit]
Beryllium chloride is used as a raw material for the electrolysis of beryllium, and as a catalyst for Friedel-Crafts reactions.
References [edit]
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- ^ Irving R. Tannenbaum "Beryllium Chloride" Inorganic Syntheses, 1957, vol. 5, p. 22. doi:10.1002/9780470132364.ch7
- ^ Cotton, F. A.; Wilkinson, G. (1980) Advanced Inorganic Chemistry John Wiley and Sons, Inc: New York, ISBN 0-471-02775-8.
- ^ Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^ Rudolph, Wolfram W.; Fischer, Dieter; Irmer, Gert; Pye, Cory C. (2009). "Hydration of Beryllium(II) in Aqueous Solutions of Common Inorganic Salts. A Combined Vibrational Spectroscopic and ab initio Molecular Orbital Study". Dalton Transactions (33): 6513. doi:10.1039/B902481F. PMID 19672497.
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN0-8493-0487-3.
- ^ Holleman, A. F.; Wiberg, E. (2001) Inorganic Chemistry Academic Press: San Diego, ISBN 0-12-352651-5
External links [edit]
- Properties of BeCl2 from NIST
Draw The Structure Of Beryllium Chloride Molecule
Source: https://en.wikipedia.org/wiki/Beryllium_chloride
Posted by: smithgert1936.blogspot.com


0 Response to "Draw The Structure Of Beryllium Chloride Molecule"
Post a Comment